van Steijn NJ et al.: Enhanced Detection and Prompt Diagnosis of Atrial Fibrillation Using Apple Watch: A Randomized Controlled Trial. JACC. 2026
https://www.jacc.org/doi/10.1016/j.jacc.2025.11.032
„Studie: AppleWatch erhöht VHF Detektionsrate 4-fach“ weiterlesen
ALONE-AF Studie
Kim D et al.: Long-Term Anticoagulation Discontinuation After Catheter Ablation for Atrial Fibrillation. The ALONE-AF Randomized Clinical Trial. JAMA 31.8.2025. doi:10.1001/jama.2025.14679
Importance Data from randomized clinical trials on a long-term anticoagulation strategy for patients after catheter-based ablation for atrial fibrillation (AF) are lacking.
Objective To evaluate whether discontinuing oral anticoagulant therapy provides superior clinical outcomes compared with continuing oral anticoagulant therapy in patients without documented atrial arrhythmia recurrence after catheter ablation for AF.
Design, Setting, and Participants A randomized clinical trial including 840 adult patients (aged 19-80 years) who were enrolled and randomized from July 28, 2020, to March 9, 2023, at 18 hospitals in South Korea. Enrolled patients had at least 1 non–sex-related stroke risk factor (determined using the CHA2DS2-VASc score [range, 0-9]) and no documented recurrence of atrial arrhythmia for at least 1 year after catheter ablation for AF. The CHA2DS2-VASc score is used as an assessment of stroke risk among patients with AF (calculated using point values for congestive heart failure, hypertension, ≥75 years of age, diabetes, stroke or transient ischemic attack, vascular disease, between 65 and 74 years of age, and sex category). The date of final follow-up was June 4, 2025.
Interventions The patients were randomly assigned in a 1:1 ratio to discontinue oral anticoagulant therapy (n = 417) or continue oral anticoagulant therapy (with direct oral anticoagulants; n = 423).
Main Outcomes and Measures The primary outcome was the first occurrence of a composite of stroke, systemic embolism, and major bleeding at 2 years. Individual components of the primary outcome (such as ischemic stroke and major bleeding) were assessed as secondary outcomes.
Results Of the 840 adults randomized, the mean age was 64 (SD, 8) years, 24.9% were women, the mean CHA2DS2-VASc score was 2.1 (SD, 1.0), and 67.6% had paroxysmal AF. At 2 years, the primary outcome occurred in 1 patient (0.3%) in the discontinue oral anticoagulant therapy group vs 8 patients (2.2%) in the continue oral anticoagulant therapy group (absolute difference, –1.9 percentage points [95% CI, −3.5 to −0.3]; P = .02). The 2-year cumulative incidence of ischemic stroke was 0.3% in the discontinue oral anticoagulant therapy group vs 0.8% in the continue oral anticoagulant therapy group (absolute difference, −0.5 percentage points [95% CI, −1.6 to 0.6]). Major bleeding occurred in 0 patients in the discontinue oral anticoagulant therapy group vs 5 patients (1.4%) in the continue oral anticoagulant therapy group (absolute difference, –1.4 percentage points [95% CI, −2.6 to −0.2]).
Conclusions and Relevance Among patients without documented atrial arrhythmia recurrence after catheter ablation for AF, discontinuing oral anticoagulant therapy resulted in a lower risk for the composite outcome of stroke, systemic embolism, and major bleeding vs continuing direct oral anticoagulant therapy.
Trial Registration ClinicalTrials.gov Identifier: NCT04432220
OCEAN Studie
Verma A et al.: Antithrombotic Therapy after Successful Catheter Ablation for Atrial Fibrillation. NEJM 8.11.2025. DOI: 10.1056/NEJMoa2509688
BACKGROUND
Whether successful catheter ablation for atrial fibrillation eliminates the need for
long-term oral anticoagulant therapy is unknown.
METHODS
We conducted an international, open-label, randomized, blinded-outcome-assess-
ment trial involving 1284 patients who had undergone successful catheter ablation
for atrial fibrillation at least 1 year earlier and had a CHA2DS2-VASc score (scores
range from 0 to 9, with higher scores indicating a higher risk of stroke) of 1 or
more (or ≥2 for women or for patients in whom vascular disease was a risk factor).
Patients were randomly assigned to receive either aspirin (at a dose of 70 to 120
mg daily, depending on availability in the local jurisdiction) or rivaroxaban (at a
dose of 15 mg) and followed for 3 years. Magnetic resonance imaging (MRI) of
the head was performed after enrollment and at 3 years. The primary outcome was
a composite of stroke, systemic embolism, or new covert embolic stroke (defined
by ≥1 new infarct measuring ≥15 mm on MRI) at 3 years.
RESULTS
A total of 641 patients were assigned to the rivaroxaban group and 643 to the aspirin
group. A primary-outcome event occurred in 5 patients (0.31 events per 100 patient-
years) in the rivaroxaban group and in 9 patients (0.66 events per 100 patient-years) in
the aspirin group (relative risk, 0.56; 95% confidence interval [CI], 0.19 to 1.65; abso-
lute risk difference at 3 years, −0.6 percentage points; 95% CI, −1.8 to 0.5; P=0.28).
New cerebral infarcts measuring less than 15 mm occurred in 22 of 568 patients
(3.9%) in the rivaroxaban group and in 26 of 590 patients (4.4%) in the aspirin group
(relative risk, 0.89; 95% CI, 0.51 to 1.55). Fatal or major bleeding (the composite
primary safety outcome) had occurred in 10 patients (1.6%) with rivaroxaban and
in 4 patients (0.6%) with aspirin (hazard ratio, 2.51; 95% CI, 0.79 to 7.95) at 3 years.
CONCLUSIONS
Among patients who had had successful catheter ablation for atrial fibrillation at
least 1 year earlier and had risk factors for stroke, treatment with rivaroxaban did
not result in a significantly lower incidence of a composite of stroke, systemic
embolism, or new covert embolic stroke than treatment with aspirin. (Funded by
Bayer and others; OCEAN ClinicalTrials.gov number, NCT02168829.)
SMARTBEATS-ALGO Studie
Fernstad J et al.: External validation of a machine learning based classification algorithm for ambulatory heart rhythm diagnostics in pericardioversion atrial fibrillation patients using smartphone photoplethysmography: the SMARTBEATS-ALGO study. EP Europace, 2025;, euaf031 (publ. 17. Feb. 2025) DOI: 10.1093/europace/euaf031
Aims The aim of this study was to perform an external validation of an automatic machine learning algorithm for heart rhythm diagnostics using smartphone photoplethysmography (PPG) recorded by patients with atrial fibrillation (AF) and atrial flutter (AFL) pericardioversion in an unsupervised ambulatory setting.
Methods and results Patients undergoing cardioversion for AF or AFL performed 1-min heart rhythm recordings peri-cardioversion at least twice daily for 4–6 weeks, using an iPhone 7 smartphone running a PPG application (CORAI Heart Monitor) simultaneously with a single-lead ECG recording (KardiaMobile). The algorithm uses support vector machines (SVM) to classify heart rhythm from smartphone-PPG. The algorithm was trained on PPG recordings made by patients in a separate cardioversion cohort. Photoplethysmography recordings in the external validation cohort were analysed by the algorithm. Diagnostic performance was calculated by comparing the heart rhythm classification output to the diagnosis from the simultaneous ECG recordings (gold standard). In total 460 patients performed 34 097 simultaneous PPG and ECG recordings, divided into 180 patients with 16 092 recordings in the training cohort and 280 patients with 18 005 recordings in the external validation cohort. Algorithm classification of the PPG recordings in the external validation cohort diagnosed AF with sensitivity, specificity and accuracy of 99.7/99.7/99.7%, and AF/AFL with sensitivity, specificity and accuracy of 99.3/99.1/99.2%.
Conclusion A machine learning based algorithm demonstrated excellent performance in diagnosing atrial fibrillation and atrial flutter from smartphone-PPG recordings in an unsupervised ambulatory setting, minimizing the need for manual review and ECG verification, in elderly cardioversion populations.
NOAH-AFNET 6 Study
Kirchhof P et al. Anticoagulation with Edoxaban in Patients with Atrial High Rate Episodes. NEJM 25 August 2023. doi: 10.1056/NEJMoa2303062
Bei Patienten mit AHRE, die durch implantierbare Geräte erkannt wurden, führte die Antikoagulation mit Edoxaban (Lixiana®) im Vergleich zu Placebo nicht zu einer signifikanten Verringerung der Inzidenz von kardiovaskulärem Tod, Schlaganfall oder systemischer Embolie, wohl aber zu einer höheren Inzidenz von Tod oder schweren Blutungen. Die Inzidenz von Schlaganfällen war in beiden Gruppen gering. Die Studie wurde deshalb wegen Sicherheitsbedenken frühzeitig abgebrochen.
„NOAH-AFNET 6 Study“ weiterlesen
Withings ScanWatch 2
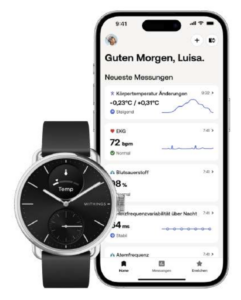
Der kombinierte Herzfrequenz- und SpO2-Sensor erfasst jetzt auch die Temperatur. Unverändert geblieben ist die Möglichkeit, jederzeit ein 1-Kanal-EKG über 30 Sekundenaufzuzeichnen, das auf iPhone, iPad, iPod touch (iOS 12 oder später) oder ein Android-Smartphone oder -Tablet mit (Android 6 oder später) übertragen werden kann. Dort erfolgen Speicherung und evtl. Weitergabe des EKG in der Health Mate App.
Die ScanWatch 2 (38 & 42 mm) wird für € 349,95 mit schwarzem Zifferblatt und silbernem Gehäuse (38 mm & 42 mm), weißem Zifferblatt und silbernem Gehäuse (38 mm) oder sandfarbenem Zifferblatt und roségoldenem Gehäuse (38 mm) angeboten. Später soll es zusätzlich Modelle mit weißem Zifferblatt und silbernen Gehäuse (42 mm) und dunkelblauem Zifferblatt mit roségoldenem Gehäuse (38 mm) geben.
Withings Move ECG

Seit September 2019 ist die Withings Move ECG der Withings France SA (2016-2018 vorübergehend Nokia Health) in Deutschland erhältlich. Es ist die erste analoge Armbanduhr mit Schrittzähler, Höhenmesser und drei integrierten Elektroden zur EKG-Aufzeichnung. Die CR2430 Knopfbatterie soll eine Laufzeit von 12 Monaten haben und leicht auszuwechseln sein. Die Uhr mit CE-Zulassung wird für 129.95 € (38 mm) in schwarz und in weiß angeboten, der Straßenpreis lag im Okt. 2020 bei ca. 116.- €.
Ein 1-Kanal-EKG über 30 Sekunden kann vom Nutzer jederzeit initiiert werde, dieses wird lokal gespeichert und bei der nächsten bestehenden Verbindung (Bluetooth Low Energy) auf iPhone, iPad, iPod touch (iOS 10 oder später) oder ein Android-Smartphone oder -Tablet mit (Android 6 oder später) in die der Health Mate App übertragen. Dort erfolgen Speicherung, EKG-Analyse hinsichtlich Vorhofflimmern und evtl. Weitergabe des EKG.
Vorderwandinfarkt: Schritt für Schritt
Infarkt oder nicht?
Schritt 1: STEMI nach Leitlinie
Schritt 2: Hochrisiko-NSTE-ACS
Schritt 3: Übrige NSTE-ACS
„Obvious STEMI“?
„Subtle STEMI“?
„Vorderwandinfarkt: Schritt für Schritt“ weiterlesen
Lewis-Ableitung
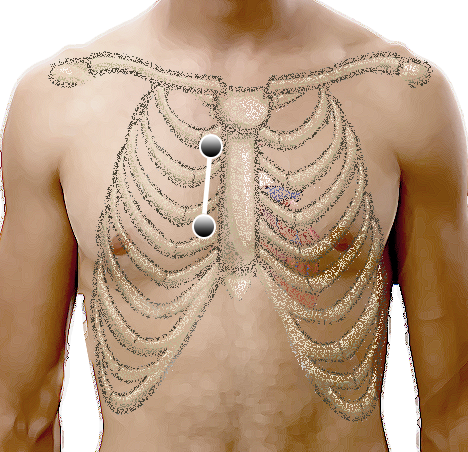
Die technische Durchführung ist einfach: Die Elektroden für den li. und re. Arm (rot und gelb) werden im 2. und 4. ICR re. parasternal angebracht. Ableitung I stellt jetzt die Lewis-Ableitung dar und sollte natürlich auch so beschriftet werden.
Abgesehen von dieser heute gebräuchlichsten Technik gibt es in der Literatur noch eine Reihe von Varianten, dazu später mehr.
„Lewis-Ableitung“ weiterlesen
Breitkomplextachykardie
Beispiel
ESC-Leitlinie 2019
AV-Dissoziation
QRS-Dauer
QRS-Achse (’no man’s land‘)
Konkordanz in den BWA
RSB-Morphologie
LSB-Morphologie
Wellens-Kriterien
Brugada-Algorithmus
Vereckei-/aVR-Algorithmus
RWPT Kriterium
Therapie
Im Folgenden geht es um regelmäßige Tachykardien, also entweder VT (ca. 80 %) oder SVT mit Schenkelblock (ca. 15 %) bzw. antegrader Leitung über eine akzessorische Bahn (ca. 5%). Bei deutlich unregelmäßigen Breitkomplextachykardien ist von Vorhofflimmern, -flattern oder einer Vorhoftachkardie mit AV-Block auszugehen.
In den letzten 50 Jahren sind diverse EKG-Kriterien und Algorithmen zur Differenzierung VT vs. SVT identifiziert und publiziert worden. Sie alle teilen das Schicksal einer sehr hohen Treffsicherheit in den jeweiligen Erstpublikationen, aber merklich geringerer diagnostischer Genauigkeit so etwa in der Größenordnung von 70-80 % bei späteren Vergleichen an anderen Kollektiven (Vereckei A 2014).
„Breitkomplextachykardie“ weiterlesen